
| Cambridge study identifies astrocyte network as new therapeutic target for Parkinson’s disease |  Preview Preview
 download download |
| Eli Lilly acquires biotech ‘newcomer’ Versanis for $2bn |  Preview Preview
 download download |
| The 10th Central and Western Forum of China Cancer Rehabilitation Organization was held — the national cancer rehabilitation was established at the meeting |  Preview Preview
 download download |
| Digital Pregnancy Rapid Test |  Preview Preview
 download download |
| Digital Ovulation Rapid Test |  Preview Preview
 download download |
| Pregnancy Rapid Test |  Preview Preview
 download download |
| Semi-quantitative Ovulation Rapid Test |  Preview Preview
 download download |
| Ovulation Rapid Test |  Preview Preview
 download download |
| DC908A Multi-Drug Test Cup Reader |  Preview Preview
 download download |
| Marijuana (THC) Saliva Test Midstream |  Preview Preview
 download download |
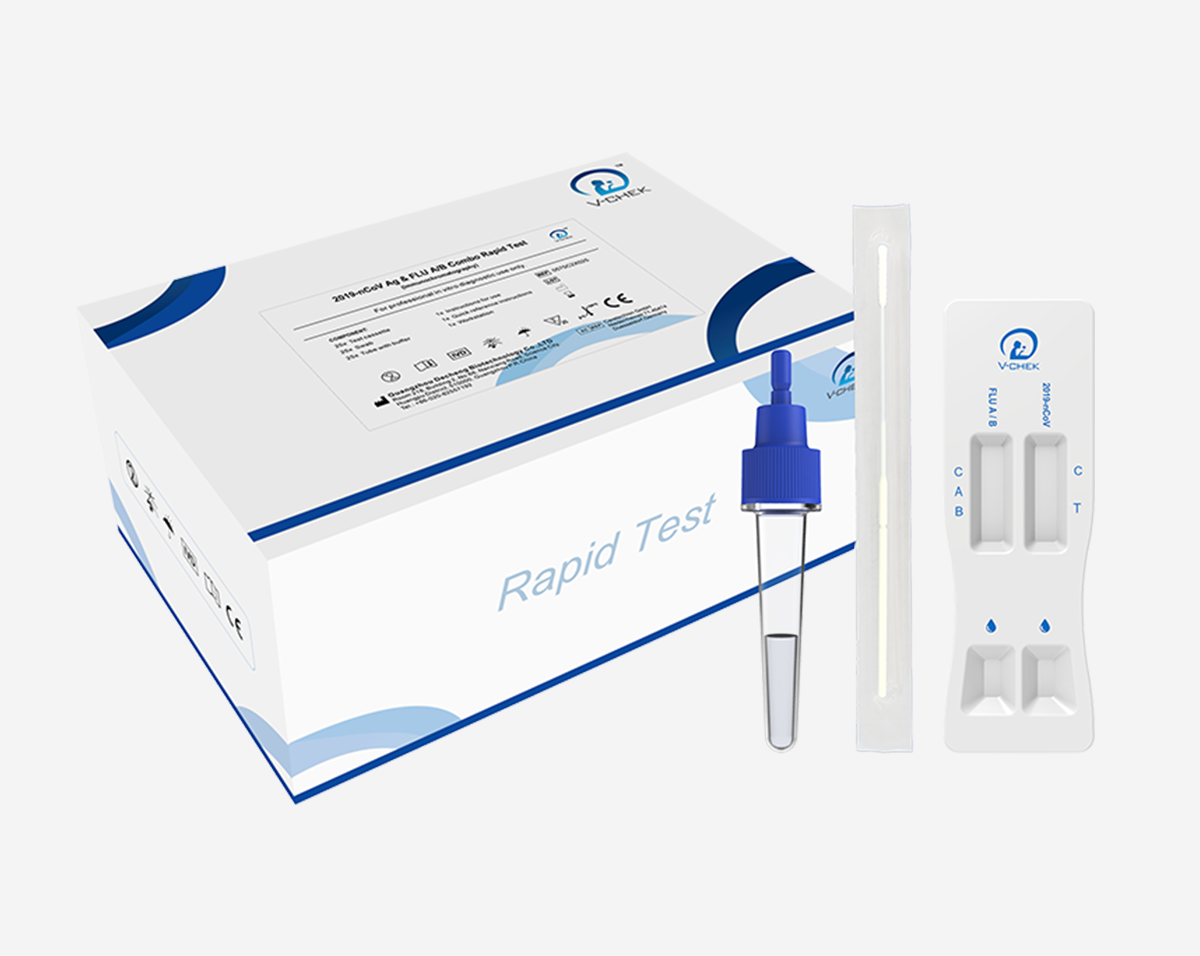

Specimen: Nasopharyngeal swab / Nasal swab
Test Format: Card
Test Time: Within 15 mins
Storage Condition: 2°C ~ 30°C (35°F ~ 86°F)
Shelf Life: 24 months
The 2019-nCoV Ag & FLU A/B Combo Rapid test is an in vitro immunochromatographic assay for the qualitative detection of 2019-nCoV antigen and influenza A / B antigens in nasopharyngeal (NP) swab specimens collected from patients with signs and symptoms of respiratory infection. This test is intended for use as an aid in the differential diagnosis of2019-nCoV and influenza A/ B viral infections in humans in conjunction with clinical and epidemiological risk factors.
This kit is for professional use only.
STORAGE AND STABILITY